SYNTHESIS • CATALYSIS • MECHANISM


[39] Ruthenium-Catalyzed Azide-Selenoalkyne Cycloadditions: A Combined Synthetic–Computational Study into Reaction Scope, Mechanism, and Origins of Regioselectivity
F. E. Bugden, A. D. Bage, A. Bajaj, B. Wang, F. J. Gibson, H. Stone, Y. Xu, F. De Proft, M. Alonso, M. D. Greenhalgh*
Adv. Synth. Catal. 2025, DOI:10.1002/adsc.70096

[38] In-Cage Recombination Facilitates the Enantioselective Organocatalytic [1,2]-Rearrangement of Allylic Ammonium Ylides
W. C. Hartley, K. Kasten, M. D. Greenhalgh, T. Feoktistova, H. B. Wise, J. M. Laddusaw, A. B. Frost, S. Ng, A. M. Z. Slawin, B. E. Bode, P. H.-Y. Cheong, A. D. Smith.
J. Am. Chem. Soc. 2025, 147, 1101–1111
* Highlighted in Synfacts

[37] Synthesis and applications of fluorinated, polyfluoroalkyl- and polyfluoroaryl-substituted 1,2,3-triazoles
F. E. Bugden, J. L. Westwood, H. Stone, Y. Xu, M. D. Greenhalgh.*
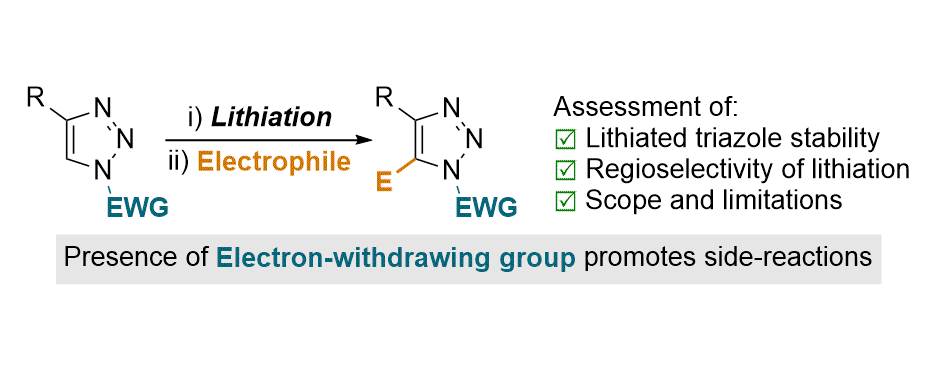
[36] Lithiation-Functionalisation of Triazoles Bearing Electron-Withdrawing N-Substituents: Challenges and Solutions
F. E. Bugden, G. J. Clarkson, M. D. Greenhalgh.*
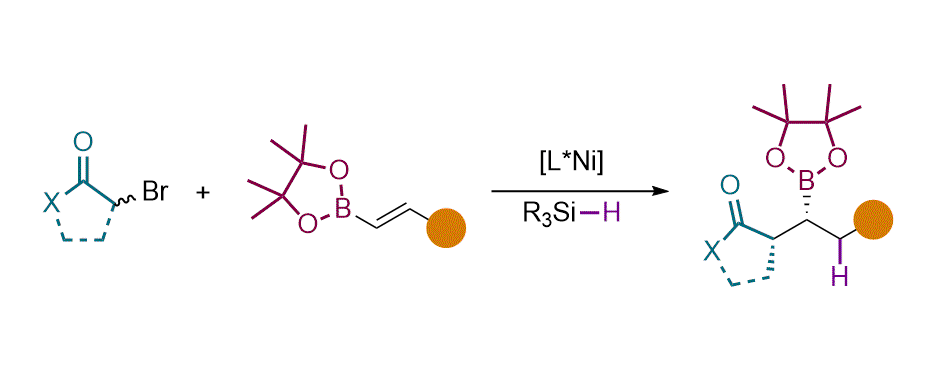
[35] Stereoselective alkyl–alkyl cross-coupling
M. D. Greenhalgh, S. P. Thomas.
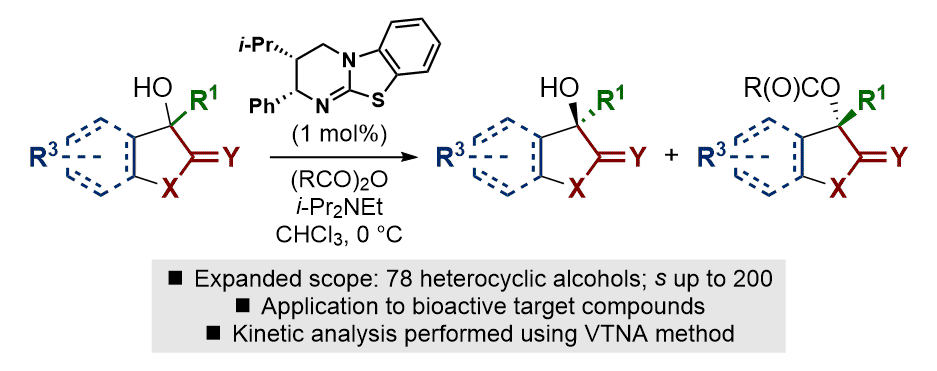
[34] Scope, Limitations and Mechanistic Analysis of the HyperBTM-Catalyzed Acylative Kinetic Resolution of Tertiary Heterocyclic Alcohols
S. M. Smith, M. D. Greenhalgh, T. Feoktistova, D. M. Walden, J. E. Taylor, D. B. Cordes, A. M. Z. Slawin, P. H.-Y. Cheong, A. D. Smith.
Eur. J. Org. Chem. 2022, 10.1002/ejoc.202101111
'Very Important Paper'
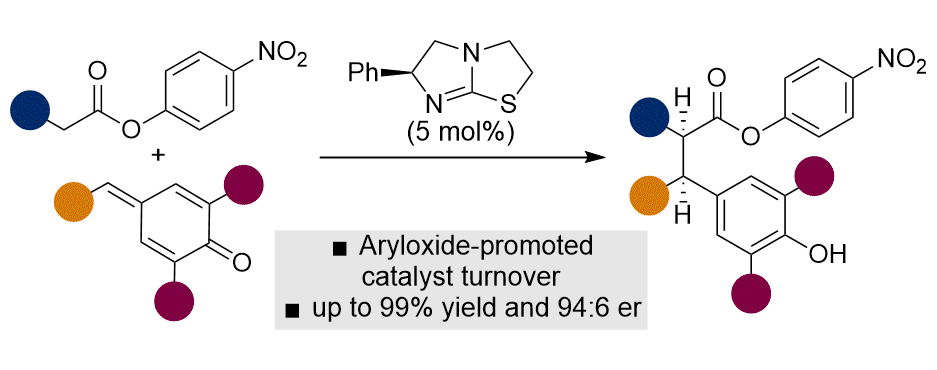
[33] Isothiourea-Catalyzed Enantioselective α-Alkylation of Esters via 1,6-Conjugate Addition to para-Quinone Methides
J. N. Arokianathar, W. C. Hartley, C. McLaughlin, M. D. Greenhalgh, D. Stead, S. Ng, A. M. Z. Slawin, A. D. Smith.
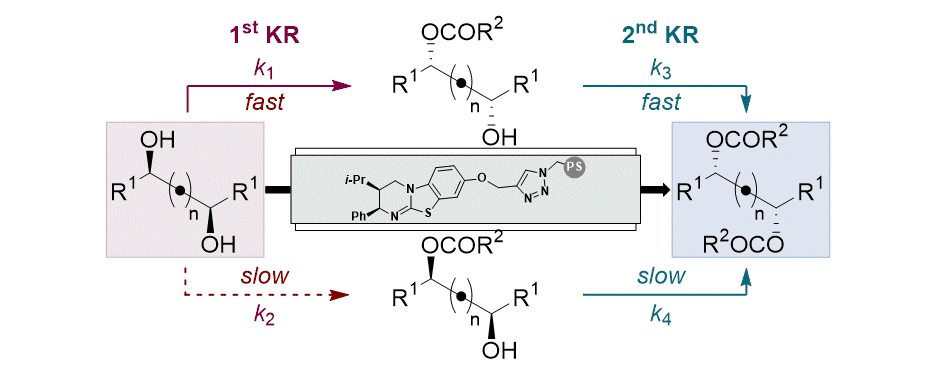
[32] Horeau Amplification in the Sequential Acylative Kinetic Resolution of (±)-1,2-Diols and (±)-1,3-Diols in Flow
A. Brandolese, M. D. Greenhalgh, T. Desrues, X. Liu, S. Qu, C. Bressy, A. D. Smith.
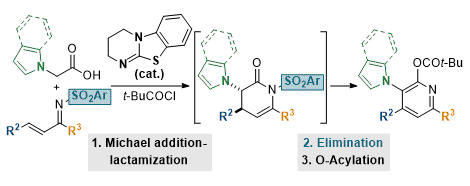
[31] Isothiourea-Catalyzed Synthesis of Pyrrole- and Indole-Functionalized Tetrasubstituted Pyridines
S. Zhang, W. C. Hartley, M. D. Greenhalgh, S. Ng, A. M. Z. Slawin, A. D. Smith.
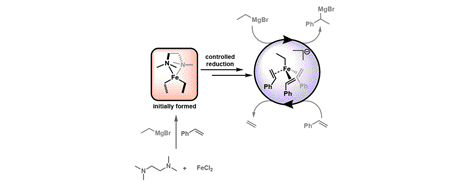
[30] TMEDA in Iron-Catalyzed Hydromagnesiation: Formation of Iron(II)-Alkyl Species for Controlled Reduction to Alkene-Stabilized Iron(0)
P. G. N. Neate, M. D. Greenhalgh, W. W. Brennessel, S. P. Thomas, M. L. Neidig.
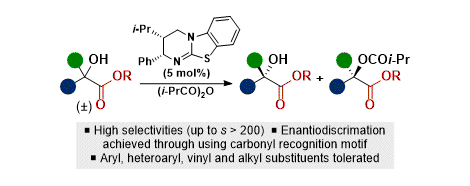
[29] Isothiourea-Catalyzed Acylative Kinetic Resolution of Tertiary α-Hydroxy Esters
S. Qu, S. M. Smith, V. Laina-Martín, R. M. Neyyappadath, M. D. Greenhalgh, A. D. Smith.
Angew. Chem. Int. Ed. 2020, 59, 16572–16578
* Highlighted in Synfacts
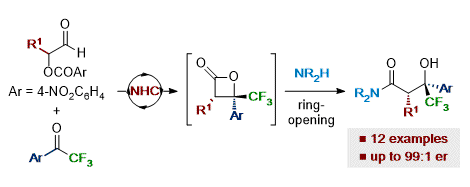
[28] NHC-Catalyzed Enantioselective Synthesis of β-Trifluoromethyl-β-hydroxyamides
A. T. Davies, M. D. Greenhalgh, A. M. Z. Slawin, A. D. Smith.
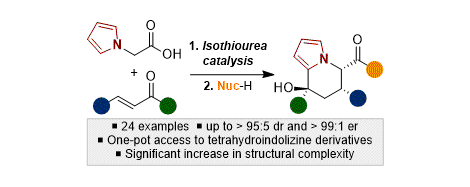
[27] Tandem Sequential Catalytic Enantioselective Synthesis of Highly-Functionalised Tetrahydroindolizine Derivatives
S. Zhang, M. D. Greenhalgh, A. M. Z. Slawin, A. D. Smith.
Chem. Sci. 2020, 11, 3885–3892
* Highlighted in Synfacts

[26] Unanticipated Silyl Transfer in Enantioselective α,β-Unsaturated Acyl Ammonium Catalysis Using Silyl Nitronates
A. Matviitsuk, M. D. Greenhalgh, J. E. Taylor, X. B. Nguyen, D. B. Cordes, A. M. Z. Slawin, D. W. Lupton, A. D. Smith.
* Highlighted in Synfacts

[25] Isothiourea-Catalysed Sequential Kinetic Resolution of Acyclic (±)-1,2-Diols
S. Harrer, M. D. Greenhalgh, R. M. Neyyappadath, A. D. Smith.
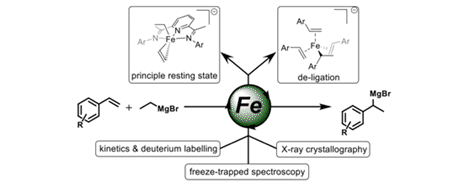
[24] Mechanism of Bis(imino)pyridine Iron-Catalyzed Hydromagnesiation of Styrene Derivatives
P. Neate, M. D. Greenhalgh, W. W. Brennessel, S. P. Thomas, M. L. Neidig.
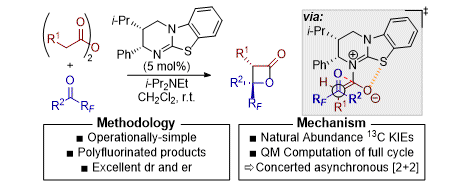
[23] Catalytic Enantioselective Synthesis of Perfluoroalkyl-Substituted β-Lactones via a Concerted Asynchronous [2+2] Cycloaddition: A Synthetic and Computational Study
D.-J. B. Antúnez, M. D. Greenhalgh, A. C. Brueckner, D. M. Walden, P. Elías-Rodríguez, P. Roberts, B. G. Young, T. H. West, A. M. Z. Slawin, P. H.-Y. Cheong, A. D. Smith.

[22] Synthesis of Fused Indoline-Cyclobutanone Derivatives via an Intramolecular [2+2] Cycloaddition
R. M. Neyyappadath, M. D. Greenhalgh, D. B. Cordes, A. M. Z. Slawin, A. D. Smith.
Eur. J. Org. Chem. 2019, 5169–5174
* Highlighted in Synfacts
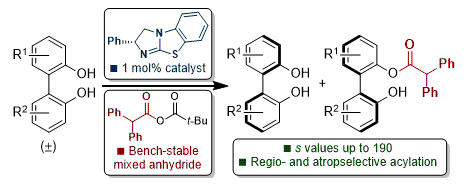
[21] Isothiourea-Catalyzed Regioselective Acylative Kinetic Resolution of Axially Chiral Biaryl Diols
S. Qu, M. D. Greenhalgh, A. D. Smith.
Chem. Eur. J. 2019, 25, 2816–2823
'Hot Paper'
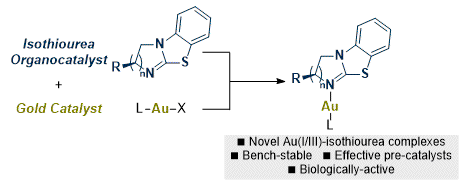
[20] Chiral Au(I)- and Au(III)-Isothiourea Complexes: Synthesis, Characterization and Application
D. Gasperini, M. D. Greenhalgh, R. Imad, S. Siddiqui, A. Malik, F. Arshad, M. I. Choudhary, A. M. A. Al-Majid, D. B. Cordes, A. M. Z. Slawin, S. P. Nolan, A. D. Smith.
Chem. Eur. J. 2019, 25, 1064–1075
'Hot Paper'

[19] Evaluating Polymer-supported Isothiourea Catalysis in Industrially-preferable Solvents for the Acylative Kinetic Resolution of Secondary and Tertiary Heterocyclic Alcohols in Batch and Flow
N. R. Guha, R. M. Neyyappadath, M. D. Greenhalgh, R. Chisholm, S. M. Smith, M. L. McEvoy, C. M. Young, C. Rodríguez-Escrich, M. A. Pericàs, G. Hähner, A. D. Smith.
[18] Best Practice Considerations for Using the Selectivity Factor, s, as a Metric for the Efficiency of Kinetic Resolutions
M. D. Greenhalgh,* J. E. Taylor, A. D. Smith.
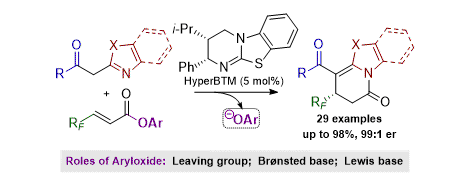
[17] Multiple Roles of Aryloxide Leaving Groups in Enantioselective Annulations Employing α,β-Unsaturated Acyl Ammonium Catalysis
M. D. Greenhalgh, S. Qu, A. M. Z. Slawin, A. D. Smith.
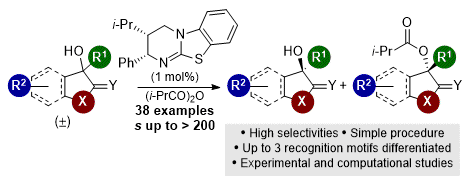
[16] A C=O•••Isothiouronium Interaction Dictates Enantiodiscrimination in Acylative Kinetic Resolution of Tertiary Heterocyclic Alcohols
M. D. Greenhalgh, S. M. Smith, D. M. Walden, J. E. Taylor, Z. Brice, E. R. T. Robinson, C. Fallan, D. B. Cordes, A. M. Z. Slawin, H. C. Richardson, M. A. Grove, P. H.-Y. Cheong, A. D. Smith.

[15] Acylative Kinetic Resolution of Alcohols Using a Recyclable Polymer-Supported Isothiourea Catalyst in Batch and Flow
R. M. Neyyappadath, R. Chisholm, M. D. Greenhalgh, C. Rodríguez-Escrich, M. A. Pericàs, G. Hähner, A. D. Smith.
* Highlighted in Synfacts
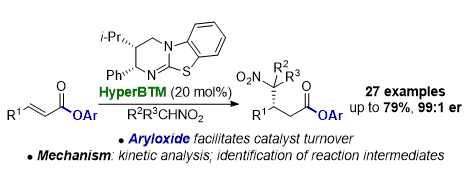
[14] Aryloxide-Facilitated Catalyst Turnover in Enantioselective α,β-Unsaturated Acyl Ammonium Catalysis
A. Matviitsuk, M. D. Greenhalgh, D.-J. B. Antúnez, A. M. Z. Slawin, A. D. Smith.
Angew. Chem. Int. Ed. 2017, 56, 12282–12287
* Highlighted in Synfacts
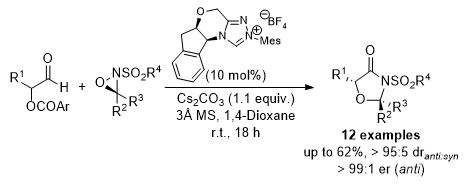
[13] Enantioselective N-Heterocyclic Carbene Catalyzed Formal [3+2] Cycloaddition Using α-Aroyloxyaldehydes and Oxaziridines
R. W. F. Kerr, M. D. Greenhalgh, A. M. Z. Slawin, P. L. Arnold, A. D. Smith.
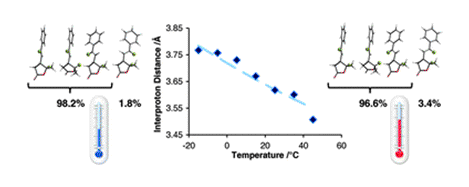
[12] Subtle Temperature-Induced Changes in Small Molecule Conformer Dynamics - Observed and Quantified by NOE Spectroscopy
C. R. Jones, M. D. Greenhalgh, J. R. Bame, T. J. Simpson, R. J. Cox, J. W. Marshall, C. P. Butts.
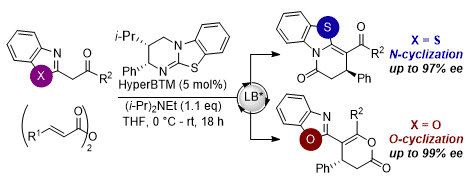
[11] Non-Bonding 1,5-S•••O Interactions Govern Chemo- and Enantioselectivity in Isothiourea-Catalyzed Annulations of Benzazoles
E. R. T. Robinson, D. M. Walden, C. Fallan, M. D. Greenhalgh, P. H.-Y. Cheong, A. D. Smith.
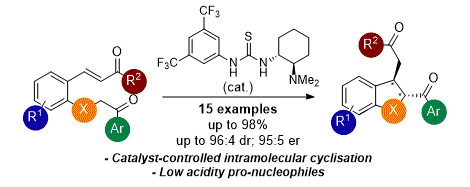
[10] Enantioselective Synthesis of 2,3-Disubstituted trans-2,3-Dihydrobenzofurans Using a Brønsted Base/Thiourea Bifunctional Catalyst
D.-J. B. Antúnez, M. D. Greenhalgh, C. Fallan, A. M. Z. Slawin, A. D. Smith.
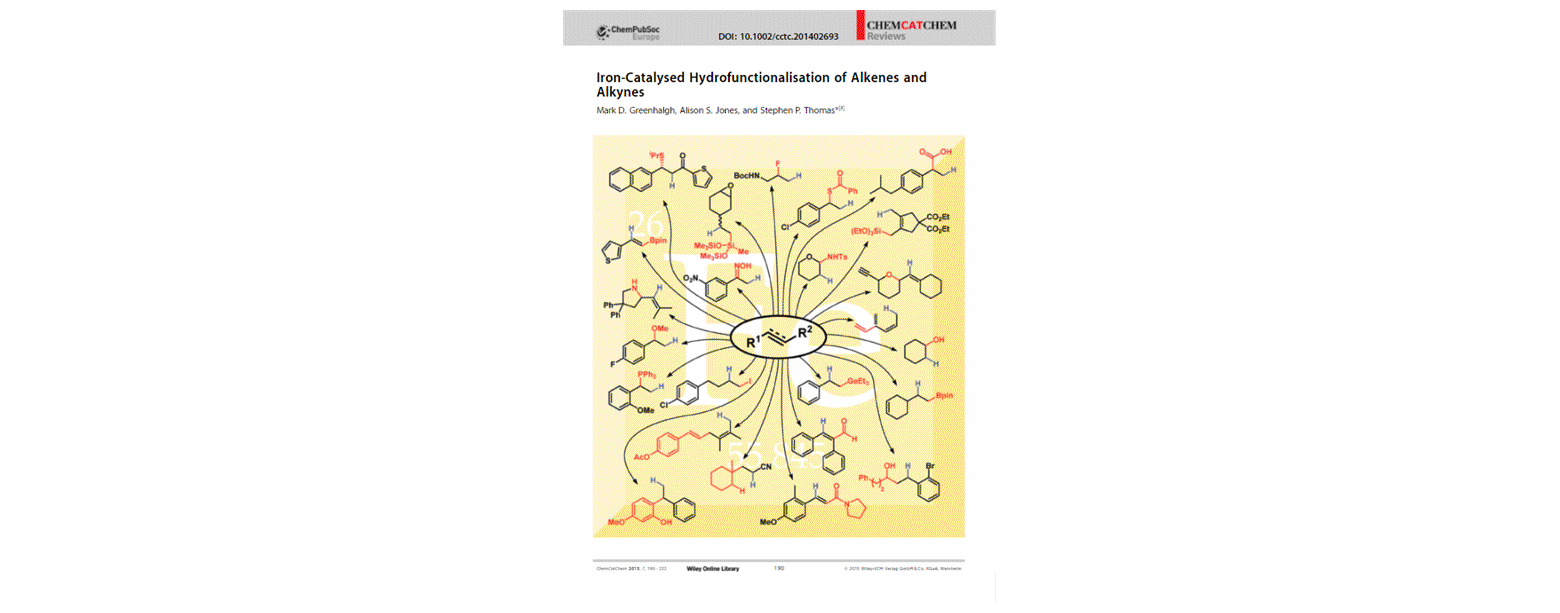
[9] Iron-Catalysed Hydrofunctionalisation of Alkenes and Alkynes
M. D. Greenhalgh, A. S. Jones, S. P. Thomas.
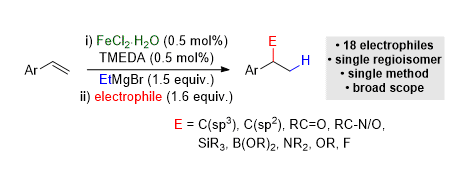
[8] Broad Scope Hydrofunctionalization of Styrene Derivatives Using Iron-Catalyzed Hydromagnesiation
A. S. Jones, J. F. Paliga, M. D. Greenhalgh, J. M. Quibell, A. Steven, S. P. Thomas.
Org. Lett. 2014, 16, 5964−5967
* Highlighted in Synfacts

[7] Iron-Catalysed Hydromagnesiation: Synthesis and Characterisation of Benzylic Grignard Reagent Intermediate and Application in the Synthesis of Ibuprofen
M. D. Greenhalgh, A. Kolodziej, F. Sinclair, S. P. Thomas.
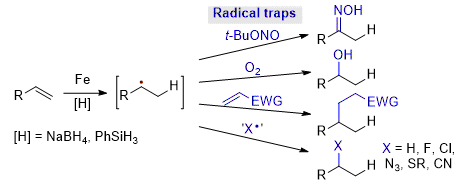
[6] Iron-Catalysed Reductive Cross-Coupling of Alkenes
M. D. Greenhalgh, S. P. Thomas.
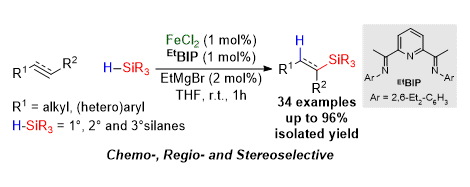
[5] Iron-Catalysed Chemo-, Regio-, and Stereoselective Hydrosilylation of Alkenes and Alkynes Using a Bench-Stable Iron(II) Pre-Catalyst
M. D. Greenhalgh, D. J. Frank, S. P. Thomas.
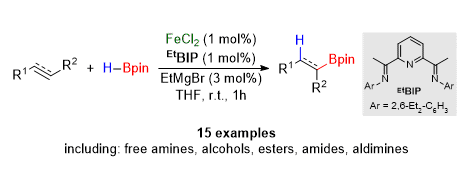
[4] Chemo-, Regio-, and Stereoselective Iron-Catalysed Hydroboration of Alkenes and Alkynes
M. D. Greenhalgh, S. P. Thomas.
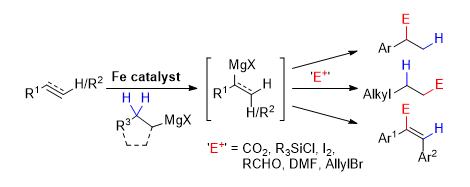
[3] Iron-Catalyzed Hydromagnesiation of Olefins
M. D. Greenhalgh, S. P. Thomas.
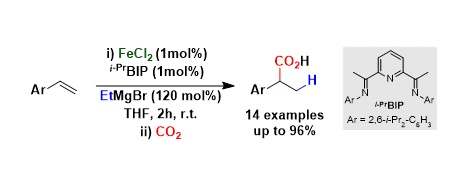
[2] Iron-Catalyzed, Highly Regioselective Synthesis of α-Aryl Carboxylic Acids from Styrene Derivatives and CO2
M. D. Greenhalgh, S. P. Thomas.
J. Am. Chem. Soc. 2012, 134, 11900–11903
* Highlighted in Synform
* Highlighted on Organic Chemistry Portal
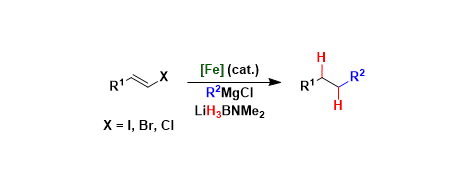
[1] Iron-Catalysed, Hydride-Mediated Reductive Cross-Coupling of Vinyl Halides and Grignard Reagents
B. A. F. Le Bailly, M. D. Greenhalgh, S. P. Thomas.